LED BY
FUNDED BY
Are you eligible?
We've designed a brief online screener with Typeform that will ask you key questions related to exclusion and inclusion criteria for the study. The Eligibility Screening survey does not collect any personally identifiable information but it does count the number of times someone looks at the questions. If your answers indicate that you might be eligible, you will see an email address that you can use to contact the research team. If you email us, we recommend that you use a personal email account — preferably one that is encrypted, such as Proton.me or Tutanota.

A study of psilocybin-assisted psychotherapy for clinicians with symptoms of depression and burnout related to frontline work in the COVID pandemic.
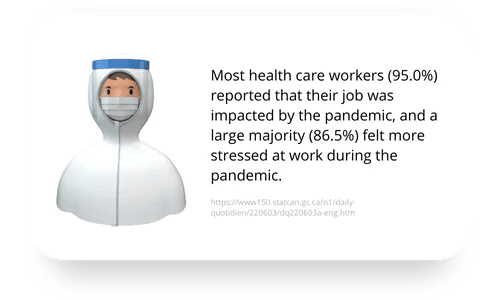
Clinicians on the frontlines of the COVID pandemic are suffering. The challenges COVID presents are substantial, and they are not over. In the meantime, psilocybin-assisted psychotherapy is showing promise for mental health conditions that overlap with symptoms clinicians are reporting related to their frontline work in the pandemic.
Although psilocybin was identified as a Schedule 1 drug in 1970, a new wave of studies beginning in the early 1990s demonstrates that psilocybin-assisted psychotherapy works differently than existing antidepressants, and the results are promising. But psilocybin-assisted psychotherapy is not yet FDA approved for any indication.
Our Perspective
We are aiming for study participants that reflect the cultural richness of clinicians who are working in the pandemic now. So we designed the study to include the people who have been right on the front lines of care–nurses, advanced practice providers, and physicians. We’ve built a study team that includes many perspectives—nurse, physician, advance practice provider, and also Black, Asian, Queer, Female, and White. For us, this is not just knee-jerk diversity—it is about doing science that is representative and more generalizable.
The Steven and Alexandra Cohen Foundation provided the primary funding for this clinical trial. Cybin, Inc., the Rita & Alex Hillman Foundation, and the Riverstyx Foundation provided additional funding for investigator training and support.
The organizations that safeguard anyone participating in this study — the FDA and the University of Washington IRB – have given us their approval. In addition, we are aware that our responsibility includes confidentiality: your identity as a study participant, or even someone who was just inquiring, is protected to the maximum degree possible. Finally, the psilocybin in this study is synthesized by the Usona Institute as a pharmaceutical grade medication.
UNIVERSITY OF WASHINGTON
CONSENT FORM
A randomized, placebo-controlled trial of psychedelic-assisted psychotherapy with single dose psilocybin for frontline clinicians experiencing COVID-related symptoms of depression and burnout
Researchers: Anthony Back MD, Principal Investigator, Department of Medicine
24-hour emergency telephone number: Anthony Back 206-619-4367
We are asking you to be in a research study. This form gives you information to help you decide whether or not to be in the study. Being in the study is voluntary. Please read this carefully. You may ask any questions about the study. Then you can decide whether or not you want to be in the study.
KEY INFORMATION ABOUT THIS STUDY
Purpose: The purpose of this study is to evaluate the effect of psychotherapy combined with a session of supervised psilocybin on symptoms of depression, burnout, and post-traumatic stress in physicians, advance practice providers, and nurses who have had these symptoms associated with frontline clinical work during the COVID pandemic.
Study duration: Participation in this study will last for a total of 7 months, from the time of the first pre-psilocybin psychotherapy session (‘preparation’) to the last questionnaires (6 months after the psilocybin or placebo medication dosing session. During that time, the preparation psychotherapy will involve about 2 weeks, the psilocybin or placebo medication dosing session will take one full day, and the followup integration psychotherapy will occur about once a week for 3 weeks. The second and third integration psychotherapy sessions and all the followup questionnaires can be completed online.
Types of activities you will do in this study: The study intervention includes 2 preparation psychotherapy sessions, which will involve discussing your experiences and the symptoms and feelings associated with them. Next will be a medication dosing session which will take place in a comfortable space that is more like a living room than a clinic room, and will take 8 hours total. Starting the following day will be 3 integration psychotherapy sessions, which will involve discussing your experiences with the medication dosing session and the feeling and insights that may have arisen. In addition to these study intervention activities, you will have screening blood tests, one urine test on the medication dosing day, questionnaires to fill out online, and brief symptom interviews.
Reasons why you might want to be in this study: You may want to be in this study to contribute to scientific knowledge about the use of psilocybin-assisted therapy for clinicians with psychological symptoms related to frontline work in the COVID pandemic. Psilocybin is a drug that is not currently approved by the FDA, and its benefit in this setting is unknown.
Reasons why you might not want to be in this study: You may wish to wait until further knowledge has been gathered about the efficacy of this treatment, or you may wish to pursue alternative treatments for psychological symptoms.
Appropriate alternative treatments: Alternative treatments would include psychotherapy, use of antidepressant medications, or possibly other medications that are used for post-traumatic stress. You would need to be evaluated by a psychologist and/or psychiatrist to determine the range of options appropriate for you.
PURPOSE OF THE STUDY
Physicians and nurses who have frontline clinical experience during the COVID-19 pandemic can have psychological issues as a result of their work, including symptoms of depression, burnout, and post-traumatic stress. This clinician COVID psychological syndrome has not been completely characterized but can be moderate to severe for some people. As yet, there are no ‘gold standard’ treatments for this clinician COVID psychological syndrome.
Psilocybin is a medication that is not currently approved by the Federal Drug Administration (FDA). Psilocybin occurs naturally in some varieties of mushrooms, but study will use synthesized psilocybin as part of a therapeutic process that involves ‘preparation’ psychotherapy for 2 sessions, a ‘medication dosing day’ when the psilocybin or placebo is given, and followup ‘integration’ psychotherapy for 3 sessions. Psilocybin acts on serotonin receptors in the brain to produce changes in how people perceive themselves and their problems that can lead to new insights. Psilocybin can also produce changes in blood pressure, heart rate, and symptoms of psychological distress such as anxiety or mood changes, although these changes are transient and generally gone in about six hours.
You are invited to participate in a research study designed to look at the use of psilocybin-assisted psychotherapy in physicians, advance practice providers, and nurses associated with frontline clinical work during the COVID pandemic who experience symptoms of depression, burnout, and post-traumatic stress. 30 subjects will be enrolled to participate in this study at the University of Washington. The participants will be randomly assigned to receive psilocybin or an active placebo, and after the 1 month evaluations are complete, the participants who received placebo will have the opportunity to receive open label psilocybin.
STUDY PROCEDURES
This section outlines the study procedures that you will experience if you decide to participate in this study. Participation in this study, including follow-up, will occur over ~ 6 months. Study visits will take place at Harborview Medical Center (HMC) in a specially outfitted clinic room, and some follow-up and self- reported questionnaires/interviews may take place remote.
Pre-study/screening:
- Screening Visit: Will take place at HMC and will take about 90 minutes.
You will be asked to come to a screening visit at Harborview Medical Center. You will sign a screening visit study consent form. If you do not already have a medical record at UW Medicine, you will need to register as a patient and a medical record will be created for the procedures at this visit.
At the screening visit, vital signs, an electrocardiogram (ECG) and a brief physical exam will be performed. You will also be asked to complete a set of self-reported questionnaires, and you will be asked a series of questions by a study clinician. You will have a blood draw (of about 4 tablespoons) that includes standard laboratory tests, and for women of childbearing age the labs will include a pregnancy test.
If you live out of state and it is not feasible for you to come to Harborview Medical Center for an in-person screening visit, or if COVID precautions make it difficult to come for an in-person visit, you have the option of utilizing a secure video communication platform such as Zoom or Skype. We will ask you to send us your most recent physical exam, ECG, and bloodwork by fax or secure electronic records transfer. The research team will review this screening information collected from this visit to determine if you are eligible. If you are eligible, you may be invited to participate in this study.
After enrollment in the study:
- Baseline questionnaires and interview will take place in a clinic room designed for therapy and will take 60-90 minutes to complete.
Following the screening visit, if it is determined that you are a good fit for the study, you will sign the separate study consent form. You will first complete a series of questionnaires that will ask questions about your experiences, your mood, and your physical and psychological symptoms. For example, the most sensitive questions include “In the last month, how much were you bothered by repeated, disturbing, and unwanted memories of the stressful experience?” and “In the past 2 weeks, I have felt a sense of dread when I think about work I have to do.”
You will also have an interview with a study clinician who will ask you questions about your psychological symptoms. In this interview, the most sensitive questions include “In the past week, do you think you have looked sad or depressed to other people? And “Have you felt tense or edgy in the past week? What about feeling fearful that something bad is about to happen?”
The information collected in the baseline questionnaires and interview will help us determine if your symptoms have improved between the screening visit and the baseline questionnaires. If they have, it is possible that you will no longer be eligible for the study, and a study coordinator will discuss this with you.
- Drug randomization
If you continue to be eligible for the study, you will be randomly assigned using a method called randomization. Randomization means that the group you are in is assigned by chance, like the flip of a coin. Your chance of receiving the study drug, psilocybin or the active placebo, niacin, is equal.
- Preparation sessions will take place in clinic room outfitted for psychotherapy and will take 60-90 minutes at two different times. The first preparation session will occur 1-2 weeks before and the second session will occur 1-2 days before the medication dosing session.
Each of the preparation psychotherapy sessions will be conducted with you and 2 study therapists who will follow you throughout the study. These preparation sessions will inform you about what to expect with the medication dosing session (which may include psilocybin or the active placebo) and will be an opportunity for you to review the experiences that you had as a physician or nurse during the COVID pandemic. The study therapists will ask questions, listen carefully, and guide you towards developing an intention for your medication dosing session, and for your recovery. At each of these preparation sessions you will be asked a short series of questions about whether you are suicidal, as a safety precaution, and if you are suicidal, you will be directed to appropriate evaluation and treatment.
The medication dosing session will take place in a clinic room outfitted for psychotherapy at Harborview Medical 1-2 days after the last preparation session. The medication dosing session will take one full-day, 8 hour session. The following will take place at this visit: You will have a urine test performed to confirm that you have not used medications other than your approved prescriptions or over-the-counter medications, and you will be given the randomly assigned study drug (psilocybin or niacin as the active placebo). If you are randomized to receive psilocybin, you will receive 25 mg. If you are randomized to receive niacin, you will receive 250 mg. During this visit, you will be in a comfortable treatment room with your 2 study therapists for the entire duration of the effects of the medication. During this session, at least one therapist will be present at all times, and both therapists will be present for most of the time. The therapists will take your blood pressure and pulse every hour. You can listen to the music provided by the study, or simply have silence. Most people do not talk that much during the medication dosing session, but you may talk as much or as little as you wish, and the therapists will follow your lead. When the medication has worn off, you will be asked to complete 2 brief questionnaires that ask about your medication dosing experience. When the study therapists feel that it is safe for you to leave, you will be discharged. You will need to arrange someone else to drive you home or to where you are staying. You will need to agree to wait for 48 hours after your release from the medication dosing session before returning to work.
We will ask for your permission to video- or audio-record the entire medication dosing session. A separate signature line for this is included at the end of this consent form. The purpose of this recording is to enable you (because you will receive a copy) and the investigators to review your experience and how the therapist interacts with you. These recordings will be kept in our locked offices in locked file cabinets or in encrypted, password-protected formats accessible only by research staff. You may choose to give permission for recording, or decline to give permission for recording, and your decision will not affect your ability to remain in the study.
- Integration sessions will take place at the same clinic room as before. You will have three integration sessions over the course of 2-3 weeks. Each integration session will last 60-90 minutes. The first integration session will occur on the day following the medication dosing session and you will have two additional integration sessions approximately every week after that. These sessions will be conducted by your two study therapists. The study therapists will ask you about your experience during the medication dosing session and will provide guidance designed to enable you to interpret what the experience might mean for you. The therapists will refrain from telling you your experiences mean—the interpretation is ultimately up to you. After each integration session, you will be asked to fill out questionnaires to assess your psychological symptoms, some of which will be identical to the questionnaires you completed in the baseline set.
The third integration session will be take place about four weeks after your medication dosing day. At this visit, you will complete another set of questionnaires, and have another 30 minute interview with a study clinician that will assess your psychological symptoms. This information will be very similar to the baseline symptom questionnaires and interview.
- Disclosure of drug group and optional open label phase
After the four week questionnaires, you will receive information about whether you received psilocybin or placebo during your medication dosing session. If you are assigned to the active placebo and after your unblinding, then you may have the opportunity of receiving the study drug, psilocybin. To receive psilocybin in an open label phase, you would complete a new set of questionnaires, symptom interview, preparation sessions, medication dosing session, and integration sessions similar to those described above. Information will be collected, and study procedures performed for subjects who select this option, and as above for the study.
- Follow-up procedures will take place remotely a 2, 3, and 6 months after your medication dosing session. This follow-up will be conducted by phone or video-conference and will take about 30 minutes to complete each time.
If you receive psilocybin, whether initially or in the open label phase, you will be asked to complete a set of study questionnaires and a symptom interview.
RISKS, STRESS, OR DISCOMFORT
We will take care to collect all of the information we need to ensure that you can safely participate in this study before initiating any of these procedures. You will not be able to participate in this study if you have an unstable medical or neurological condition, or if your psychiatric condition is sufficiently concerning that the psychiatrists associated with our clinic think it would be unsafe or unwise for you to participate in this study.
Interruptions in existing treatments or delay in seeking other treatments
In order to participate in this study, you may have to change any treatments you are currently receiving for your symptoms. In particular, if you are taking any antidepressant medication, you will need to be tapered off that medication prior to entering the study, and you may experience worsening of symptoms during that period. In order to minimize this risk, you will be able to contact a study physician 24 hours a day should any problems arise.
In addition, you will need to refrain from starting any new antidepressant medications that act on the serotonin system in the body during the entire study period, until you have completed the questionnaires and symptom interview that occur four weeks after the medication dosing session. The study physicians will make exceptions for worsening symptoms or emergencies.
Your participation in the study will be terminated immediately if your condition significantly worsens, especially if you feel suicidal.
Safety Plan for Suicidality: During all study visits a Masters or Doctoral level clinician will assess your well-being – responses, intent, plan, etc. while administering the C-SSRS (Columbia Suicidality Severity Response Scale, which all clinicians have received training on) and if there is a serious concern they will either ask you to meet with or call the PI, or report to the clinic (if the study visit is being done remotely), or go to the nearest ER, or call 911. If necessary, we will also call your treating clinician and/or your emergency contact. As part of the consent, you are required to designate an emergency contact person and provide your treating clinician’s contact information. We will coordinate with your treating clinician in regards to local plan for such emergencies so our study team can follow this plan should an emergency situation arise. The study investigators will take steps to reduce and manage any physical or psychological changes that could be unpleasant or harmful.
Risks of screening and baseline questionnaires, and symptom interviews
The risks of screening include any stress or discomfort associated with a brief physical examination or answering questions that may be sensitive in the questionnaires or the symptom interview. You may find answering these questions to be inconvenient, uncomfortable, or upsetting. The symptom interviews may include some personal questions about previous experiences. You will be able to complete these activities in a private room, and you may decline to answer any questions that make you uncomfortable. You can discuss any concerns with someone on the research staff, and you will have access to clinicians trained to address these issues. You may take short breaks if you need them.
Risks of having an electrocardiogram (ECG)
Sometimes the adhesive pads used to attach the leads for recording the electrical activity of the heart can cause skin irritation. Such irritation usually clears without treatment. Should you develop a skin reaction, study staff will assist you in finding the necessary treatment.
Risks of having a blood draw
We will need to draw blood for routine laboratory testing. The baseline labs are necessary to review your current health prior to participation. The risks or side effects associated with taking blood from a vein are bruises, local irritation (swelling) with itching, slight bleeding and inflammation. In rare cases, it may result in thrombosis (blood clots) or an infection. Insertion of the needle can cause localized pain or pain at the needle puncture site. Subjects may feel slightly weak or lightheaded, or faint. Occasionally, in rare cases, inserting the needle can result in injury to a nerve. We minimize this risk by having skilled nurses and phlebotomists do our blood drawing. In the very rare event of a puncture-site infection, the study staff will aid you in treating the site.
Risks of preparation sessions
The risks of the preparation sessions are that the study therapists may ask personal questions that evoke personal feelings from your past, or feelings that may still be present, and this may be uncomfortable or unpleasant. These sessions will take place in a private room, or over a secure video connection, and you may decline to answer any questions or stop the sessions at any time.
Risks of medication dosing sessions, including psilocybin
The psilocybin used in this study is manufactured at the Usona Institute, which provides researchers with pharmaceutical grade synthesized psilocybin. You will be given one tablet of either Psilocybin or the active placebo, niacin. The dose of psilocybin you will
be receiving in this study is 25 mg.
Common acute side effects of psilocybin are almost all psychological, and include anxiety, changes in thought (experiencing thinking speeding up or slowing down), changes in motion perception, changes in time perception (time slowing down or speeding up), depersonalization (feeling as if one is “outside oneself”), derealization (feeling as if the world is unreal or as if one is “in a dream”), dizziness, fatigue, impaired concentration, inattention, mood lability (rapid and sometimes profound changes in mood), nausea, nervousness, parasthesias (strange bodily sensations or feelings), perceptual alterations (general), altered time perception, alteration in visual perception (distortions, illusions and imagery seen with eyes open or closed), and unusual thought or feelings about the self or the world. Most of these effects are acute and last no longer than six hours. For the most part, people receiving psilocybin maintained insight concerning the nature and source of their experience. However, some participants occasionally exhibited reactions including paranoid ideation or temporarily lost insight into the experimental situation. In recent studies, these reactions have been managed with reassurance and breathing directed by the study therapist. However, if medication is required the study physician may perform an assessment and prescribe a small dose of lorazepam.
The physiological effects of psilocybin include pupillary dilation and detectable but moderate increases in blood pressure or heart rate. In other studies, changes in blood pressure and heart rate have sometimes occurred at only at one point in time. These effects are transient and are generally gone approximately six hours after drug administration. People in previous human studies have tolerated doses of psilocybin equal to or greater than the doses employed in this study.
The most likely potential adverse effect of psilocybin is anxiety, which is usually handled by the study therapists. Uncommon but more serious side effects of psilocybin include panic, delusion, and cognitive impairments, which have mostly occurred with doses of psilocybin higher than used in this study. If these side effects occur, clinical evaluation will be given with the potential for medication to be given if needed.
A rare risk reported in anecdotal literature but not in a research study with psilocybin in the past 5 years is that a participant could have a brief psychotic reaction. If this were to happen, clinical evaluation and treatment will be given. Another rare risk is you may have visual disturbances that you first notice during the medication dosing day that reoccur at lower intensity long after the medication has left your body, including halos, distorted colors, or bright lights. This condition is called Hallucinogen Persisting Perception Disorder, and is not a sign or precursor of psychosis, but you should see your physician.
All the acute side effects of psilocybin explained in this consent form are transient and generally gone approximately six hours after drug administration.
A subacute side effect of psilocybin that has been reported in the literature is mild insomnia for the first night or two after the medication dosing session. This side effect has been described as self-limiting. If needed you may take over the counter sleeping aids if this occurs. In the unusual case where insomnia persists, study staff can direct you to a clinician for further evaluation.
At the end of the medication dosing session, you must be medically cleared to be discharged, and you must be driven home, either by a driver arranged by you or by the site personnel, which could include a ride-sharing service or a taxi. You may return to work not earlier than 48 hours after the medication dosing session is over.
Because this treatment is considered investigational and has not received FDA approval, there may be risks that we do not know about at this time.
Risks of receiving niacin (active placebo)
The single administration and dosage of niacin being used in this study, 250 mg, is often associated with mild, expected physiological side effects which is why it is used as an “active placebo”. These acute side effects include flushing, tingling, itching, and redness of the face, arms, and chest. Other minor include upset stomach, intestinal gas, mild transient headache, and mild dizziness, which are transient and resolve on their own.
Pregnancy risks
WOMEN PLEASE NOTE: You should not participate in this study if you are pregnant, or might become pregnant during the period of this study, or are breast-feeding. You will take a pregnancy test as part of the study tests. If your test result is positive, you will not be included in this study. We will discuss with you the need to avoid becoming pregnant during this study and what precautions you plan to take. If you change your mind about becoming pregnant or your method of avoiding pregnancy, we will ask you to notify us immediately. You will be asked to take a urine pregnancy test the day of the drug administration.
Risks of integration sessions
The risks of the integration sessions are that the study therapists may ask questions that evoke uncomfortable or unpleasant memories from your medication dosing session or your past experiences prior to the medication dosing session. These sessions will take place in a private room, or over a secure video connection, and you may decline to answer any questions or stop the sessions at any time.
ALTERNATIVES TO TAKING PART IN THIS STUDY
If you choose not to part in this study, alternatives to treat symptoms of depression, burnout, and post-traumatic stress include psychotherapy and medication treatment with FDA approved antidepressants. You would need to have an evaluation by a psychologist and/or a psychiatrist to determine the best options for you.
BENEFITS OF THE STUDY
You may experience reduced symptoms of burnout and depression as a result of psilocybin-assisted psychotherapy, or placebo-assisted psychotherapy, but because this is the first study of this therapy for this condition, it is unclear how effective psilocybin-assisted psychotherapy will be. You may not experience direct benefits from this study. Your participation may benefit others in the future. We hope that the knowledge gained from this study will lead to better treatments for physicians and nurses with this condition.
SOURCE OF FUNDING
The University of Washington is receiving the study drugs (psilocybin and niacin) from the Usona Institute, and funding to conduct the study from the Steven and Alexandra Cohen Foundation.
CONFIDENTIALITY OF RESEARCH INFORMATION
All identifiable information that is obtained in connection with this research study will remain confidential as described below. Information about the study visit, including information about your laboratory results, blood draws, and urine test. Your medical record will not identify you as a participant in this study. The link between your identifiers and the research data will be destroyed after the records retention period required by state and/or federal law. Information about you will be kept in locked offices, on password-protected computers, or in secure clinic files on a University of Washington secure server; or in locked cabinets on a locked unit. Only the members of the research staff will know your identity. Any identifiable information that is obtained in connection with this study will remain confidential and will be disclosed only with your permission or as required by U.S. or State law.
If we learn that you intend to harm yourself or others, we must report that to the authorities.
The U.S. Food and Drug Administration (FDA) reserves the right to review study data that may contain identifying information.
The Usona Institute, a nonprofit research drug company that is providing the psilocybin, reserves the right to review study data that may contain identifying information.
A description of this clinical trial will be available on http://www.clinicaltrials.gov, as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time.
Your participation in this study will be noted in your UW medical record.
USE OF INFORMATION AND SPECIMENS
Returning Results to You
You will have the opportunity to receive any results from research tests that are clinically relevant, which would include results of blood draws, ECG, or urine test.
Using Your Data in Future Research
The information that we obtain from you for this study might be used for future studies. We may remove anything that might identify you from the information. If we do so, that information may then be used for future research studies or given to another investigator without getting additional permission from you. It is also possible that in the future we may want to use or share study information that might identify you. If we do, a review board will decide whether or not we need to get additional permission from you.
OTHER INFORMATION
You may refuse to participate and you are free to withdraw from this study at any time without penalty or loss of benefits to which you are otherwise entitled. If you wish to withdraw, please contact the researcher listed on page 1 of this consent form. You may be withdrawn from the study if there is evidence of suicidal ideation and will be referred to appropriate psychiatric care. You may also be withdrawn at the beginning of the study, if after the baseline interview and questionnaires, it is determined your symptoms have improved between the screening visit and baseline.
All costs of study-related procedures will be billed to the research team. The study will pay for visits that require onsite procedures at HMC.
RESEARCH-RELATED INJURY
If you think you have a medical problem or illness related to this research, contact Anthony Back MD (206-619-4367) right away. He will treat you or refer you for treatment.
For a life-threatening problem, call 911 right away or seek help immediately. Contact Anthony Back MD (206-619-4367) when the medical emergency is over or as soon as you can.
If you are injured as a result of being in this study, necessary medical treatment will be offered at a UW Medicine facility.
The costs of the treatment may be billed to you or your health insurance just like other medical costs, or it may be covered by the UW’s discretionary Human Subjects Assistance Program (HSAP), depending on a number of factors. The researcher may request HSAP coverage by following established procedures. If you wish to request HSAP coverage yourself, contact the researcher or the UW Human Subjects Division at hsdinfo@uw.edu or 206-543-0098. You may also call collect to the UW Human Subjects Division at 206-221-5940 if you do not otherwise have access to a telephone. Ask the researcher if you would like information about the limits and conditions of the HSAP. The UW does not normally provide any other form of compensation for injury. However, the law may allow you to seek payment for injury-related expenses if they are caused by malpractice or the fault of the researchers. You do not waive any right to seek payment by signing this consent form.
Printed name of study staff obtaining consent Signature Date
Subject’s statement
This study has been explained to me. I volunteer to take part in this research. I have had a chance to ask questions. If I have questions later about the research, or if I have been harmed by participating in this study, I can contact one of the researchers listed on the first page of this consent form. If I have questions about my rights as a research subject, I can call the Human Subjects Division at (206) 543-0098 or call collect at (206) 221-5940. I will receive a copy of this consent form.
Printed name of subject Signature of subject Date
Copies to: Researcher
Subject
Video - Audio Recording (option): We are requesting your permission to video/audio record some of your participation as part of this study. If you agree to the record, we will use these recordings of your medication dosing session for evaluating the quality of the therapists’ work, and for improving future interventions. We would like to record the drug administration session in entirety. In addition, we would like your permission to keep these recordings for the purposes of research and our own team training purposes indefinitely. The recording is optional. You may choose to give permission for one or both uses of the recordings, or you may decide not to participate in recording at all. Your decision will not affect your ability to remain in the study.
If you agree to participate, we will keep the recordings in our locked offices in locked file cabinets, accessible only by the research staff. To protect your confidentiality, we will date and then code the recordings with a study identification number (rather than your name) and session numbers.
These coded tapes will be kept along with the study records, either indefinitely (with your permission) or for no more than four years after the study ends.
I agree that video/audio recording may be taken of me as part of this study. The recordings may be used for (initial all that apply):
- any purpose relevant to research, medical evaluation, training
(Initial)
- purposes of the study only
(Initial)
Seeking permission to retain tapes for future use: You have the choice of how long we may keep your tapes.
Please read our Privacy Policy.
How Do I Register?
Download > Start > Complete Walkthrough > Sign Up
Step 1. First Download the Quantified Citizen App here
Step 2. Complete the following walkthrough and select "Sign Up" to create an account:




Note: To skip the walkthrough select "Already have an account? Sign in" at the bottom.
Step 3. For your privacy we identify your account with 12 random words. Take note of these words and store them in a safe place. Treat these like you would treat a password.

Note: You do have access to these words inside the app using the dropdown hamburger menu in the top left and selecting "My Account".
Step 4. Select the the box to confirm that you agree to our Terms and Privacy Policy
Step 5. Select the "SIGN UP" button to continue.
Step 6. Enter a 4 digit password and then re-enter again to confirm. This can be used to sign in. You can also opt for Apple's Face ID after entering the 4 digit password.
Step 7. You should have a Completed screen as shown below.
Step 8. Press "Next" to enter your dashboard.
What happens to my data?
All of your data is completely anonymous. When you use the Quantified Citizen app you are the owner of your own personal information.
Rest assured all of your data is completely anonymous. When you use the Quantified Citizen app you are the owner of your own personal information. By using our services we do not have ownership, right, title, or interest in your personal information.
Do I receive my personal data during or after a study?
Not yet, but could be coming soon!
Currently, no personal data is shared during or after a study. This has been a popular request from the Quantified Citizen community and we are making strides to make this possible!
Quantified Citizen does not control the data of the customer (you). In order to keep your data anonymous and secure we are not permitted to transmit any customer data to any third party companies or publish it without explicit written consent. You can read more in our Privacy Policy.
✅ Wellness Together Canada: Call 1-866-585-0445
This is a free, confidential, 24/7 hotline providing free mental health and substance use support. Their platform offers various services ranging from basic wellness information, to one-on-one sessions with a counselor, to participating in a community of support. Whatever it is you’re looking for, visit https://wellnesstogether.ca.
✅ Crisis Text Line Canada: Text “HOME” to 686868 or visit https://crisistextline.ca
Crisis Text Line USA: Text “HOME” to 741741 or visit https://crisistextline.org
These are 24-hour, free, support lines (via text medium) for people experiencing all kinds of crises, including suicidal thoughts.
✅ National Suicide Prevention Lifeline (USA): Call 800-273-TALK (8255)
This is a 24-hour, toll-free, confidential suicide prevention hotline available to anyone in suicidal crisis or emotional distress. For Spanish (Español), dial 1-888-628-9454. You can also use the Lifeline Chat on the web https://suicidepreventionlifeline.org/chat.
✅ Substance Abuse and Mental Health Services Administration National Helpline (USA): Call 800-662-HELP (4357)
This is a free, confidential, 24/7, 365-day-a-year treatment referral and information service (in English and Spanish) for individuals and families facing mental health and/or substance use challenges.
✅ Fireside Project (USA): Call or text (3pm to 3am PST) 62-FIRESIDE > 623-473-7433
This is a peer support for those in the midst of psychedelic experiences, those sitting for others, and those integrating past psychedelic experiences. You can also access support via an app: https://firesideproject.org/app.
✅ Psychedelic Support Therapy
This an online directory of experienced providers, information about integration groups/circles, and online education in the psychedelic space, for more information, visit https://psychedelic.support.
✅ Organization of Psychedelic and Entheogenic Nurses
This organization represents nurses, at all levels of training, who work with patients utilizing therapeutic psychedelic medicines. They offer a list of clinical resources for those seeking integration and harm reduction, as well as psychedelic societies/communities, and professional organizations. If you want to learn more, visit https://openurses.org/clinical-resources.